Liquid-liquid phase transitions
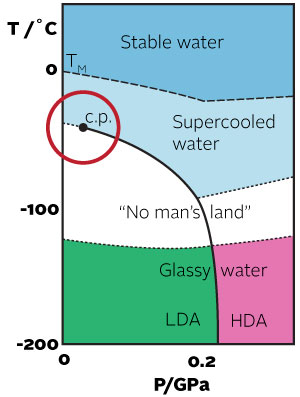
Liquid water and its complex crystallisation and vitrification behaviour has been studied for at least 235 years. Despite extensive study, many of the strange properties of water – such as its density maximum at 4° C or its increasing heat capacity on cooling the liquid below the melting point – remain unexplained.  In the 1970s, Speedy and Angell suggested that the presence of a second critical point at ~220 K could explain many of the odd behaviours of water. Unfortunately, this second critical point (if it exists) is difficult to study as it lies below the homogeneous nucleation temperature in a region known as "no man's land". A number of recent studies of nanoconfined water have suggested that water does indeed undergo a transition at 220-230 K from fragile (strongly glass forming) to strong (near Arrhenius behavior). Although, these studies have generated considerable controversy, there is evidence to believe that the influence of the second critical point extends all the way into the normal liquid region up to the boiling point.
In the 1970s, Speedy and Angell suggested that the presence of a second critical point at ~220 K could explain many of the odd behaviours of water. Unfortunately, this second critical point (if it exists) is difficult to study as it lies below the homogeneous nucleation temperature in a region known as "no man's land". A number of recent studies of nanoconfined water have suggested that water does indeed undergo a transition at 220-230 K from fragile (strongly glass forming) to strong (near Arrhenius behavior). Although, these studies have generated considerable controversy, there is evidence to believe that the influence of the second critical point extends all the way into the normal liquid region up to the boiling point.
We are using a number of techniques to study liquid-liquid transitions, ranging from femtosecond optical Kerr effect (OKE) spectroscopy to ultraslow microsocpy.
This work is performed with a number of international collaborators.

Animation of a liquid-liquid transition in triphenyl phosphite (TPP) taking place through nucleation as observed through phase-contrast microscopy (Joanna Mosses, 2013).